The objective of WP1 for the first year is first to develop sensor system chemistries ready for lablet integration. Two approaches exploring pH sensors for gold-surface functionalisation, and configurable nanoparticle assembly ready for surface integration. One approach, using a switchable organic system involving four distinct states that can be interconverted using both pH and redox chemistry as control parameters has been developed. The key molecules involved in this system are the phenanthridine-based heterocycles; 1-isobutyl-1,2,3,12b-tetrahydro-imidazo[1,2-f]phenanthridine (TIP) and 5-(2-isobutylamino-ethyl)-phenanthridinium (AEP). These two states are interchangeable using pH control, and in addition can also be further manipulated by oxidation or reduction to convert them to their 'pH-inert' forms; 1-isobutyl-2,3-dihydro-1H-imidazo[1,2-f]phenanthridinium (DIP) and 5-(2-isobutylamino-ethyl)-5,6-dihydro-phenanthridine (AEDP), respectively. UV and 1H NMR experiments carried out in a biphasic DCM/water solution were used for in situ structure determination. The results showed that the pH modulated cyclisation and phase transfer process between the TIP and AEP states was essentially quantitative and repeatable without any significant loss in activity and that reduction or oxidation could be used to lock out these states against such acid-base induced changes, Figure 1. Furthermore we are able to mount these onto gold nanoparticles made in a bespoke microwave reaction system allowing the programmable assembly of differently sized particles, Figure 2.
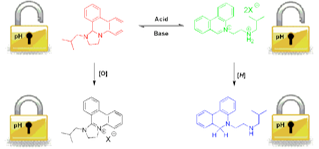
Figure 1 Scheme of our molecular pH / redox locking system sensor