Gene-Container coupling using PNA
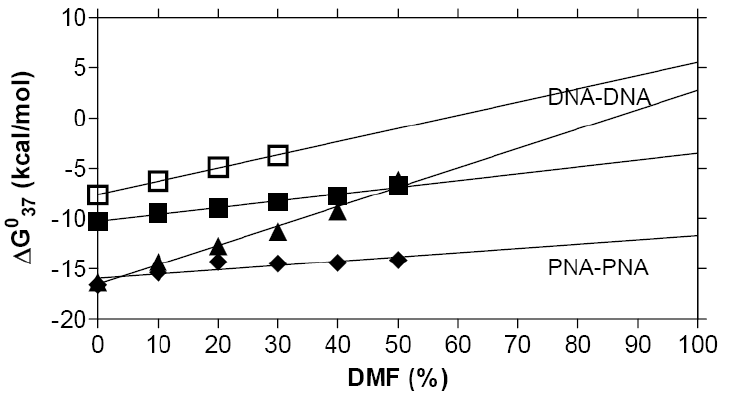
Although we have not yet succeeded in having PNA oligomers freely soluble in a lipid phase, a series of experiments using high concentrations of organic solvents (50-70 % dimethylformamide or dioxane), and extrapolating these results to zero water content (Fig. 2) has allowed the prediction that PNA duplexes, in contrast to DNA duplexes will indeed be stable in organic solvents [64]. Furthermore, these data somewhat surprisingly also indicate that the fidelity of sequence recognition (sequence discrimination) is not significantly affected either.
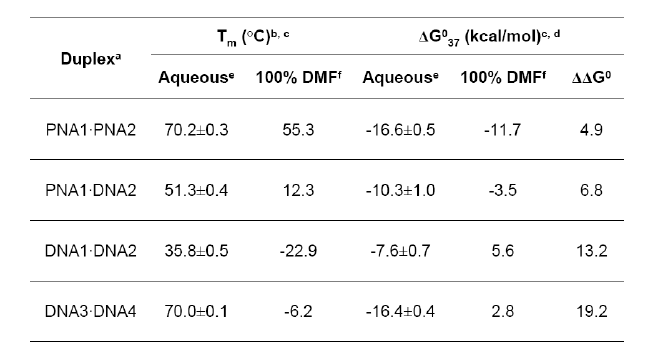
Figure 2: a) Gibbs free energy of hybridization of DNA-DNA, DNA-PNA
and PNA-PNA duplexes vs. organic solvent strength, indicating the
highest relative
stability for PNA-PNA duplexes, both in aquaeous and organic solvent.
Melting temperatures and numerical values are given in b).
Efforts for direct physical
incorporation (association) of the template
with the lipid phase have involved chemically modified PNAs either
within the backbone (for embedding/dissolution in the lipid phase) or
by chemical conjugation of lipophilic groups. To this end PNA decamers
containing multiple leucine modified backbone units or decanoyl-lysine
modified backbone units (Fig. 3) have been
synthesized and their solubility properties analyzed in a traditional
water-octanol partition system. Of these the leucine modified PNA
duplex
(6 out of twenty residues) partitioned more than 99% into the water
phase,
whereas preliminary data indicate that the octanoyl-lysine modified PNA
duplex
(4 out of twenty residues) distributes equally between the two phases.
Therefore, the latter type of modification may eventually lead to PNA
oligomers that are fully incorporated into a lipid environment.
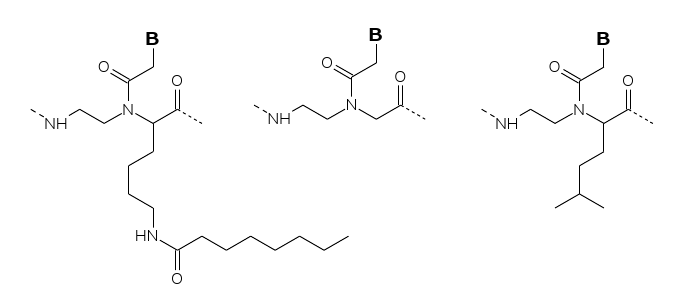
Figure 3: PNA conjugates with various lipophilicities (various
lengths
of attached fatty acids).
A series of fluorescein PNA fatty
acid conjugates with varying lipophilicity has been synthesized
(Fig. 3). These are expected to
associate with lipid droplets and vesicles at the surface, having their
lipophilic tail embedded in the lipid phase and the PNA part exposed
into the aqueous phase. Studies of the interaction of these with lipid
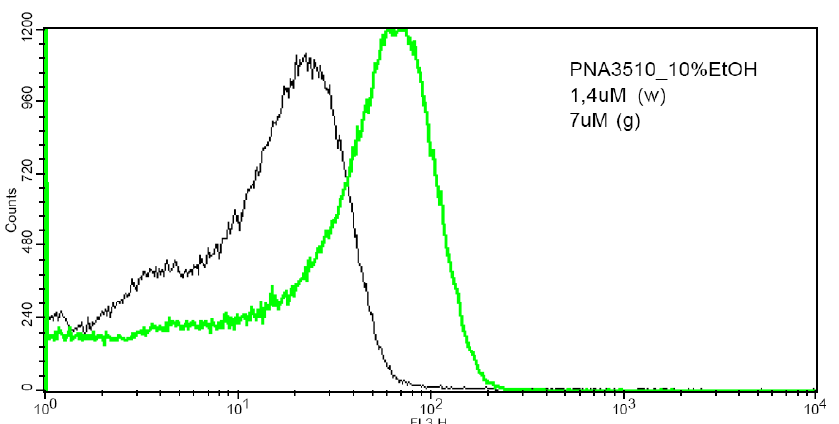
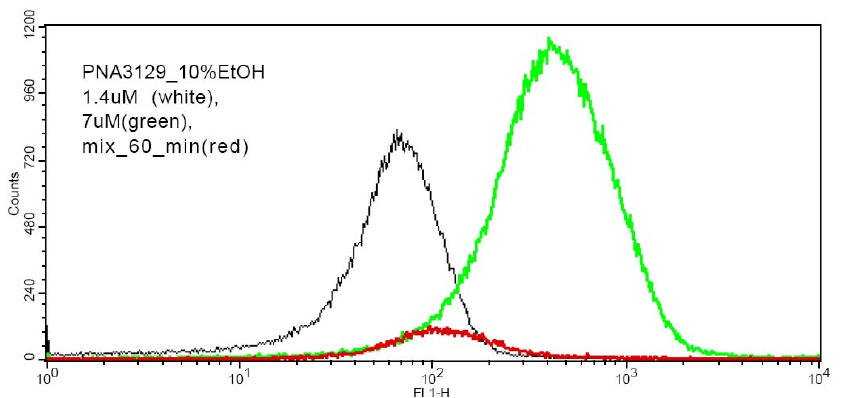
vesicles have been initiated using FACS (fluorescence assisted cell
sorting) (Fig. 4), and the results show that
such lipid PNA conjugates are indeed effectively bound to lipid
vesicles, and that the binding kinetics in terms of off rates may
be measured by this technique.
Figure 4: Fluorescence measurements from a FACS machine of population of 1 um POPC vesicles with attached fluorescent PNAs. a) Dose-response signal b) Mixing of two populations (vesicles with low PNA + vesicles with high PNA content) results in an intermediate population. The technique enables measurement of PNA lifetimes within vesicles (60 min in this case).