Towards the analysis of PNA self-replication via kinetic 19F-NMR titrations
In order to monitor autonomous PNA self-replication via 19F-NMR signals of product and precursors, PNAs containing hydrophobic isosteres [56] of thymine and uracil (F-PNAs) were synthesized. While the synthesis of Fmoc/Bhoc protected nucleobase monomers was carried out as described previously [57], an efficient way for the synthesis of F-PNA monomers was elaborated. We found a new and improved route [58] to synthesize 2,4-difluoro-5-methyl-phenylacetic acid (3a) and converted the product into the PNA building block 7a. 2,4-Difluoro-phenylacetic acid (3b) was prepared analogously and the corresponding monomer 7b was obtained in good yield. In addition PNA building block 7c was synthesized starting from commercially available 2-fluoro-4-trifluoromethyl-phenylacetic acid (4c), because the trifluoro-methyl-group is expected to provide different NMR shift shifting properties. Figs. 36 and 37 summarize the synthesis of F-PNA monomers 7a-c.
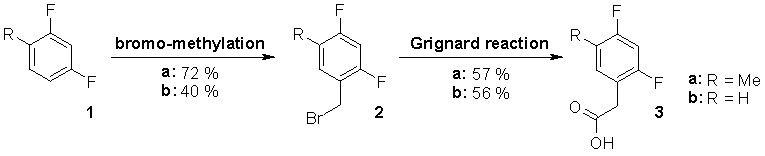
Figure 36
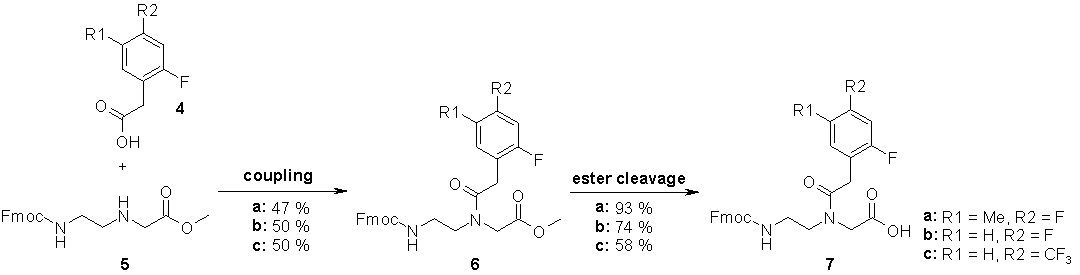
Figure 37
A palindromic PNA sequence 8 was designed in such a way that the end modifications provide water solubility and are not reactive in EDC mediated ligation reactions. PNA hexamers 8a and 8b were prepared by automated solid phase synthesis, purified by RP-HPLC and analyzed by MALDI-TOF-MS.
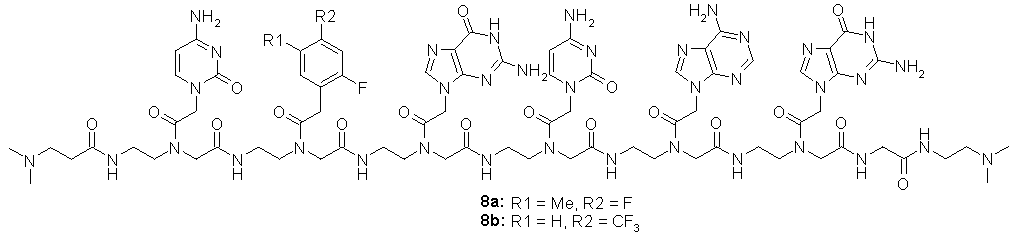
Figure 38
Since template 8a is already available in quantities needed for NMR-experiments, our effort is now concentrated on preparing template building blocks 9 and 10 by liquid phase synthesis.
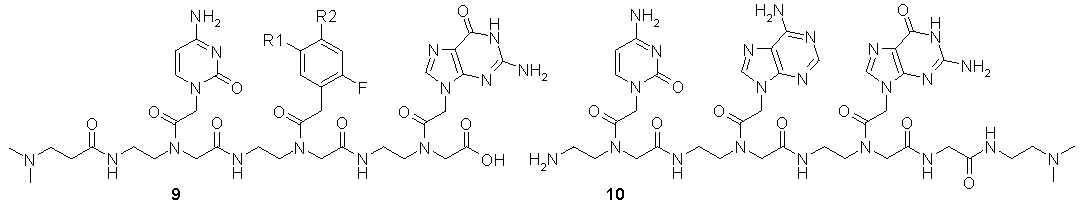
Figure 39




