Redox-controllable DNA ligation using 3'-5' disulfide formation (closed to the public)
In an electronically programmable microfluidic environment, electron uptake and removal may be viewed as the simplest conceivable way to implement a metabolism. If ligation were achievable by a redox-reaction, spatially controlled microelectrode chemistry could be coupled to the formation or destruction of template molecules. We thus aimed at the development of an activator independent chemical DNA ligation or replication system and to combine it with a suitable analytical method like FRET or fluorescence quenching for monitoring in microfluidic systems. We choose thiol-disulfide exchange reaction as ligation chemistry and an Alexa488-Dabcyl pair for ligation detection. For synthesis of 3'- and 5'-thiol modified oligonucleotides 5'-thio-desoxycytidine and 3'-thio-desoxyguanosine nucleosides were synthesized by Mitsunobu reaction introducing a thioacetate.[41] The conveniently protected thiol-precursors were immobilized on thiol-CPG respectively (Figure 29). The building blocks were successfully introduced in automated standard and reversed oligonucleotide synthesis.
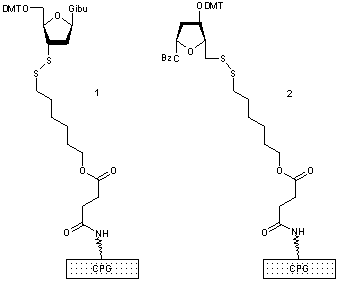
Figure 29




