Smart drug delivery systems [1]
When developing drug delivery systems the main problem is how to
overcome body barriers (skin, gastrointestinal
epithelium, blood-brain). Typically, targets are membranes (interfaces)
and membrane receptors, which, having the hydrophobic interior, put
constraints on the drugs which in turn makes drugs difficult to
transport through blood. In addition, promising hydrophils/hydrophobes
drugs can be solid or
easily degraded, hence need for encapsulation for protection, or
formulation enabling controlled, retarded release.
Lipids and lipid encapsulation are ideal for drug delivery systems:
they are amphiphilic (operate at interfaces), self-assemble to
encloses
hydrophilic core, have temperature induced porosity, are biodegradable
and
easily modifiable. They are the stuff of which biological interfacial
barriers are
made, and hence nature's own technology is used to mediate cell
environments; lipids themselves can act as drugs or prodrugs
influencing cell function and signalling, e.g., cubic and hexagonal
lipid structures are much more viscous and can cause much
slower release of drugs (local anesthetics).
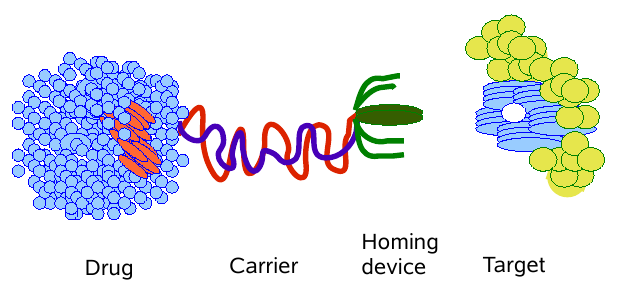
"Magic bullet" as a synonym for a perfect drug was coined by Paul
Erlich, at the begining of 20th
century. The idea is to selectively target and kill the diseased tissue
without affecting healthy cells. Designing the three components - drug,
carrier, homing device attaching
to a target, Fig.1 - has proven to be much more diffcult to achieve
than at
first thought.
It might seem at first that ordinary liposomes (vesicles), with
encapsulated drugs, should be good candidates, but they have a short
lifetime in the blood
due to immune response, causing early release and degradation of drugs
in
the blood. To prevent this, the so-called stealth liposomes have been
designed with polymeric extensions on the lipids with
hydrophilic heads. These protrusions cause a repelling entropic force
(between the individual liposomes and between liposomes and the cells
of immune system) and make a watery outer
shell which make the liposomes invisible. The two effects cause
liposomes' much longer lifetime in the blood [1].
A direction to explore are PNA-vesicle aggregates where PNA, bound onto
the vesicle, acts at the same time as a homing device
and a drug (e.g., inhibiton of translation process within a cell due to
the stronger PNA-DNA
binding) . See also sequence tagging.
Figure 1: Schematic of a "magic bullet".