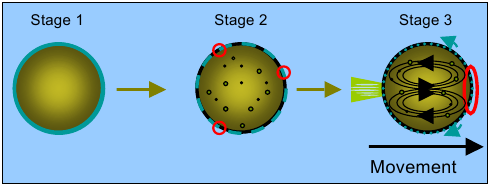
From chemotaxis towards soft robots
In our efforts to understand the
dynamic properties of self assembled matter applied towards creating a
protocell, we discovered a very simple chemical system that exhibits
movement. The movement presents itself as autonomous or directional
depending on the experimental conditions. In this way our simple
artificial system displays a critical feature of living systems, namely
chemotaxis.
For the future development of this technology, we envision application in the field of soft robotics. This will involve using the oil droplets (or similar) as nano-and microscale actuators for very small robots. This soft matter robot technology will advance the state of the art in robotics with regard to form, ease of manufacture, range of application, cost, robustness, and integration into the environment. The development of microscale agents capable of locomotion through a chemical language will become the state of the art with regard to the future development a new kind of chemically embodied intelligence with impact as well in cognitive sciences and biology.