Real-time evolution of protocells in microfluidic computers
Microfluidic computers
The state-of-the-art of lab-on-a-chip technology developed at PACE
is programmable
microfluidics which adds the functionalities of computer-controlled
flows (via programmable electrodes and actuators), and real-time
monitoring (via processed read-outs from within the system), [3].
Microflows and
bio-chemical reactions can now be not only controlled
and programmed, e.g., in/out of a chemical microreactor, but also
feed-back adjusted, based on e.g. fluorescent intensity of chosen
reactants. The platform enables a completely new paradigm of chemical
processing, and is appropriately termed microfluidic computers [4], or
chemical Turing machines, likely to become the future lab-robot
technicians in biochemical sciences [5].
The highly spatially
resolved
microfluidic networks integrated with feed-back programmable
flow-control units present then a remarkable experimental platform in
stark contrast to the ordinary epruvette/beaker/tube systems. In
particular, evolution of bio-molecular interactions involving various
nucleic-acid molecules, lipids, etc. can be monitored and/or programmed
in sequential and parallel ways not previously possible. This in turn
opens avenues for realizing both natural and artificial
metabolic-information pathways within microfluidic-based structures.
Moreover, it enables the life-support of entirely new living systems -
protocells - that present a transition from nonliving to living matter
[6].
Evolution on a chip [4,7]
In-vitro enzyme independent molecular evolution has two main problems not fully resolved: (1) a robust and evolvable replication with a high yield, and (2) a build-up of more complex molecular interactions, both of which seem to be crucial traits of living entities. The strength of microfluidic computers is their ability to optimize individual processes (e.g. only self-replication) via loop operations, but also the possibility of optimizing evolution of more complex couplings between different chemical systems (e.g. genetic and metabolic). For example, two initially decoupled processes, self-replication and metabolism, can be integrated within a microfluidic computer into a synergistic gene-metabolism cycle characteristic for minimal protocells. The idea is to evolve and select the protocells both for their replication as well as their metabolic abilities, or in other words, to increase the complexity of replicators by assisting them to do more than just replicate - in this case catalyze a metabolism, Fig.3.
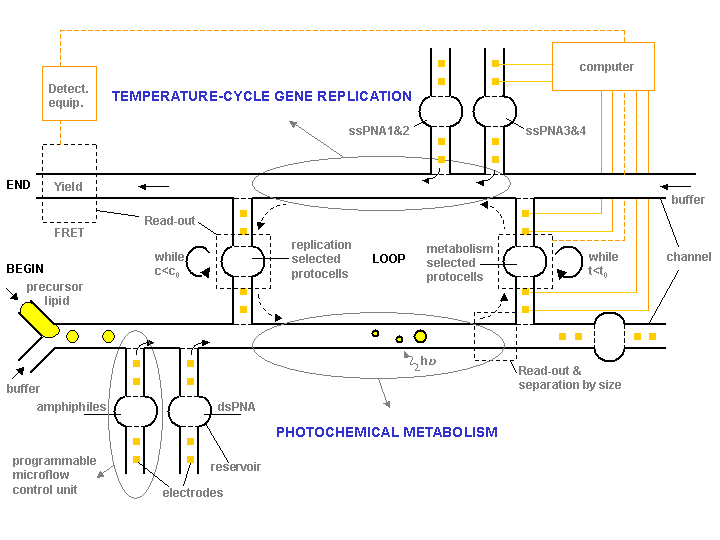
Figure. 3: Schematic of a microfluidic computer operating on “evolvable hardware”; the idea is to co-evolve metabolic (lower channel) and genetic (upper channel) functionalities of protocells. Such systems could provide initial life-support for protocells and a controlled evolution. In the long term we believe that such systems can be utilized to “program” protocells for useful tasks such as to grow in particular chemical environments.




