Catalipids: Towards a minimal chemoton (closed to the public)
Catalipids are synthetic lipids containing a catalytic moiety such
as an imidazole unit. Imidazole derivatives are known to act as
acid-base catalysts as well as nucleophilic catalysts of acyl- or
phosphoryl transfer reactions. In PACE, the development of catalipids
and their investigation in nonenzymatic self replication was greatly
inspired by the Ganti's chemoton theory (1.1).
In our concept for a minimal chemoton (1.2),
we thought to couple the chemical self replication (1.2.1)
of an oligonucleotide template to the generation of a lipid molecule
released from the reactive catalipid conjugate in the termolecular
complex:
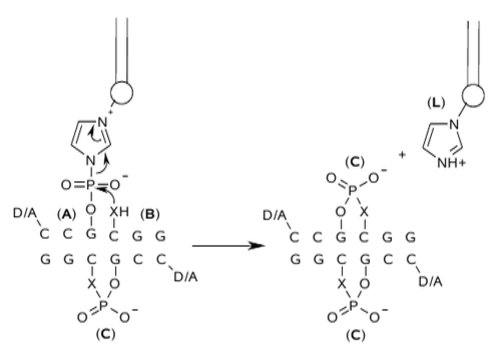
Figure 2 Ligation
reaction via phosphoimidazolide
activation, a imidazole-containing lipid acts as leaving group.
We started with model reactions on the role of the imidazole part and deciphered the kinetics of metabolic chemistry (1.3), when a phosphate group is activated by a water soluble carbodiimide as a fuel in the presence of an imidazole derivative as a catalyst. The reaction network involves phosphate activation by the carbodiimide, hydrolysis of the activated phosphate, transformation into a phosphorimidazolide, the latter's hydrolysis, and further reactions and was studied by NMR kinetics followed by dynamic modelling using our SimFit program. We found that phosphorimidazolides are formed rapidly, undergo the desired reactions while being less sensitive against hydrolysis compared to the primary carbodiimide adducts.
As the self-replicating component we selected an oligonucleotide replicator and our "mixed labeling" FRET-detection (1.4) scheme previously described.
We developed a number of different methods for the synthesis of catalipids (1.5) and studied the formation of giant unilamellar vesicles (1.6). As a result of these studies we found catalipid structures and GUV-formation conditions compatible with the conditions needed for template replication. The carbodiimide driven formation of reactive catalipid phosphorimidazolide conjugates (1.7) proved however quite difficult. In the aggregated state catalipids did not react at all. At elevated temperatures conjugation did occur but it was hampered by carbodiimide hydrolysis and side reactions due to increased reactivity. Only after shortening the lipophilic tail (1.8) we were able to arrive at reactive conjugates which finally allowed the study of ligation reactions with catalipid release (1.9). With lipophilic chain lenghts in the order of six to ten carbon atoms we expect that the crosstalk of self replication and compartiment formation might be in the micellar regime where again NMR studies could be helpful to gain insight. As the fluorine nucleus is highly sensitive to changes in its supramolecular environment we have now developed catalipids with fluorine label (1.10). FRET and NMR-experiments on the kinetic analysis of such crosstalk will take several years to arrive at a level of insight that is available for non-coupled (isolated) subsystems.
In summary, the creation of chemoton-like machinery in which the self-replication of a template is stoichiometrically coupled to the formation of compartiments is far more difficult than expected. Progrss was achieved, but a completion of this work goes beyond PACE.
- Redox-driven self-replication via isosteric 3'-5'-disulphide formation (closed to the public)
- Nano-sized container-scaffolds via trisoligo self-assembly
- Systems Chemistry and PACE
- Kinetic NMR titrations: A tool to analyze self-replicating systems
- Towards the analysis of PNA self-replication via kinetic 19F-NMR titrations




