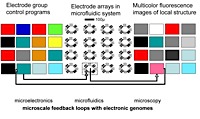
While the achievement of chemical autonomy in cellular functions remains a key goal of artificial cell research, cellular functions can potentially also be complemented by a microsystem having a comparable physical size. Complementation means that certain cellular functions are supported or taken over by the microsystem.
Microfluidic systems provide a broad repertoire of solutions for physical complementation (cf. Table below). They are on the right scale for complementing artificial cells on a 1-on-1 individual basis; they have electronic interfaces to digital computers; they allow a high density, quasi-planar optical interface; they are evolvable via reconfiguration; they have a natural extension to nanofluidics.
Microfluidic complementation options
- Supply of buffer, nutrients and/or energy.
- Removal of waste.
- Local concentration cycling of molecules.
- External provision of (some) catalysts.
- External provision of informational molecules.
- Surface immobilization.
- Regulated temperature and thermo cycling.
- Physical containment and isolation.
- Selective separation of molecules (artificial membranes).
- Phase control: e.g. hydrophobic patterning.
- Support system for structured multi-step chemistry.
- Support system for parallel screening: channel networks.
- Material transport between cells and regulated cell division.
One simple example is containment, where electric fields generated by electrodes can potentially restrain the dilution of genetic material or nutrients to the environment. Further microsystem containment approaches we have employed include the use of phase boundaries, including those of hydrogels and oils, as well as optical, magnetic and mechanical restraints. Another example is using micro-flows to create directed nutrient and waste fluxes which simplify for the cell the task of accumulating resources and removing wastes. Actually, the range of possibilities is much larger, including catalytically active complementation and the use of active separation technologies. Part of the richness of microsystem complementation comes from the possibilities of creating multi-compartmental cells, with dedicated chemistries in separate locations, and directed transport mediating the exchange of material between the compartments.
We are especially interested in microflow systems, rather than open surface microstructures, despite exciting recent developments, because of their ability to support long term homeostasis in chemical systems. Complementation brings the additional advantage of providing a means of programming artificial cells: the choice of complementation can direct the spatial structure, the resource structure, the metabolism and/or the replication cycle in specific directions.
Evolutionary complementation
The complementation of protocells can be regarded analogously to the complementation by “music minus one” electronic media that provides soloists with a complete orchestral accompaniment with the solo part missing. A programmable complementation system can consecutively reduce the level of support, increasing the degree of autonomous orchestration of the chemical system. In principle, a smooth succession of environmental challenges can be provided, easing the combinatorial search for viable artificial cells down to manageable steps, starting from a fully supported system in which all aspects of the protocell are under microfluidic control. Of course, the difficulty of the individual steps is dependent not only on the innate evolutionary potential of the chemical system at each stage, but also on the aptness of engineered changes to its composition. In addition, and perhaps less obviously, a combinatorial succession or array of different environments can be used to enhance the effective evolvability of the chemical system, especially at early stages.
A withdrawal scenario might involve, a sequence of small changes in the
a) duration of barrier electronic fields,
b) confining geometry, and flow rates for supply channels,
c) quantities of active and inactive chemical precursors supplied externally,
accompanied by combinatorial search for PNA or catalipids which can fulfill the functions a-c above. Although the step to membrane formation, for instance, may be a difficult one, requiring very specific genetic amphiphiles, at least for compatibility with self-replication, it could be broken up into separate optimization steps for the subtasks a and b, or intermediate steps via micelle forming amphiphiles, starting with the fully supported artificial cell.