In a closed homogenous chemical system, prepared in an out-of-equilibrium state, all information is initially in the form of chemical information, Kchem, corresponding to an initial amount of exergy. Chemical reactions consume the exergy, according to the 2nd law of thermodynamics, and the chemical information thus decays. If spatial structure is built up, we find that some of the information, at least temporarily, is transformed to spatial information, Kspatial. If the system remains closed, though, such spatial structures cannot remain and the system approaches a homogenous equiliobrium state. In an open system, a steady inflow of chemical energy may keep the chemical information at a high level, and spatial structures may be supported. First we will discuss the closed system and derive a set of information flow quantities, and in the next section the system will be open for molecular flows across the boundary which will result in additional effects. So to start with we neglect the flow term B in Eqs. (16) and (18).
Let us discuss briefly some thermodynamic characteristics of the system. The chemical system, described by the dynamics Eq. (16) but excluding the result of the in- and out-flow (which will be treated separately), results in a thermodynamic entropy production s (in units of Boltzmann’s constant) according to
![]() . (19)
. (19)
The entropy production is determined by one term corresponding to the entropy produced due to diffusion within the system and one term given by the reactions that tend to even out the chemical non-equilibrium in the system. The entropy production certainly leads to a decay of the information in the system — decay of structural information as well as of chemical information.
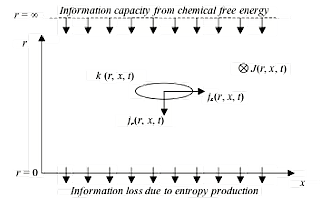
We shall make an information-theoretic description of how information is flowing in the system that connects to the thermodynamic loss of information due to entropy production. This will be formulated in a continuity equation for information density k(r, x, t), taking into account flows both in scale (r) and in space (x), see figure below.
In the limit of r –> ∞ we cannot distinguish any spatial structure, but the chemical information Kchem is still present, unaffected by the resolution parameter. In a decomposition of the total information this part can therefore be considered as present at the r –> ∞ limit. The chemical information will be consumed by the chemical reactions and we should therefore expect that a proper definition of information flow shows how information will flow in the direction towards smaller length scales r. If the system gives rise to spatial structure, that should be captured in the continuity equation, resulting in a temporal accumulation of structural information.
Figure 2. A schematic picture of information (capacity) flows in a chemical pattern formation system. The pattern is characterised by an information density k(r, x, t) distributed over spatial dimensions as well as over different length scales r. Information flows both in space and in scale, where the flow is destroyed when it gets down to the microscopic level. Here information disappears into microscopic degrees of freedom due to entropy production. Information capacity enters the system at the very large scale due to a diffusion-controlled inflow of chemical information or Gibbs free energy. A pattern is formed when information that flows downwards in scale is aggregated at certain positions as described by the continuity equation.
It is reasonable to think that information is leaving the system, through the thermodynamic entropy production, at the finest length scales of the system, i.e., at r = 0. At this point information leaves the macroscopic description that we have of our system, and the information is spread out on microscopic degrees of freedom. Therefore, we define the information flow jr(r, x, t) in the direction of smaller r, at the border r = 0, to be equal to the chemical entropy production,
![]() . (20)
. (20)
To define this flow for general resolution values r, we generalise by introducing the resolution operator into the expression for entropy production,
![]() . (21)
. (21)
In the limit of r –> ∞, this information flow can be written
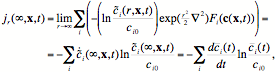 (22)
(22)
where we have used the dynamics, Eq. (18), and Eq. (11). Note that this expression is equal to the decay of chemical information, –dkchem/dt, since
![]() , (23)
, (23)
since Si dci/dt = 0 due to the normalisation. In general it holds that dkchem/dt < 0 (except for extreme cases), which means that there is a positive flow of information jr(r, x, t) at the infinite length scale limit in the direction of smaller length scales, similar to what we have at the other limit, r = 0. The interpretation is then that information flows from its origin as chemical information down through the length scales, temporarily halting if spatial structure is built up, but sooner or later continuing to the finest length scale where it disappears as entropy production.