We shall use Eq. (1) as a starting point for our combined information-theoretic and thermodynamic analysis of the system. Since homogeneity is assumed to be broken, we introduce spatially varying concentrations ci(x), expressed in the probabilistic form pi(x) = Vci(x)/N. (We assume that the number of molecules per unit volume, N/V, does not depend on position.) This results in probability distributions pi(x) over the different molecules that are normalised at each position. Then, Eq. (1) is replaced by
![]() , (2)
, (2)
where the information K is now an integral over Kullback information quantities for each position in the system,
![]() . (3)
. (3)
We shall use the average concentration within the system, defined by
![]() , (4)
, (4)
in order to decompose the total information K in Eq. (2) into two terms, one that quantifies the deviation of the average concentrations from equilibrium, Kchem, and one that quantifies the deviation from homogeneity, i.e., the presence of spatial structure, Kspatial,
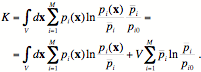 (5)
(5)
Therefore we define the spatial information, Kspatial,
![]() , (6)
, (6)
and the chemical information, Kchem,
![]() , (7)
, (7)
and we can write the total information K as
![]() . (8)
. (8)




