Compartmentalization is an organization principle widely used in nature. On one hand, eukaryotic cells contain various vesicle-like sub-compartments (such as nucleus, endosomes, mitochondria, etc.). On the other hand, tissues can be understood as compositions of membrane-bound processing units (cells). The reasons for this compartmentalization are manifold and partially caused by the fact that natural systems are the result of evolutionary processes and not of rational planning. Some aspects, however, are rooted in chemistry and physics and are also of importance in artificial systems: The implementation of a complex reaction network, for example, is difficult if one is only allowed to use one reaction container (e.g. one single vesicle). This for the following reasons:
- Individual steps of the reaction may interact with each other in an undesired manner.
- Many process steps only run efficiently under relatively specific conditions, say with respect to pH. These conditions may vary quite considerably for the different steps. Combining all steps in one reaction container implies to choose "one fits for all" conditions, which may lead to a significantly sub-optimal result.
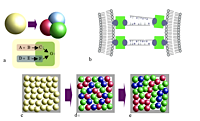
Compartmentalization facilitates the de-coupling and optimization of different process steps. But there is a price to pay: The products of the individual steps have to be transported between the vesicles. Modern process design relies on the usage of multiple reaction vessels, which are connected such that they form a finely tuned process chain. It is exactly this process chain that we want to realize by the spatial structuring of the vesicles. Exchange of material between vesicles happens either via pores between adjacent (hemi-fused) vesicles or via diffusion. This material exchange is maximal between nearby vesicles. Indeed, already vesicles which are randomly placed on a substrate may yield considerable "compartmentalization gains" (Fig. 1a,c,d); by employing the selective coupling of vesicles to the substrate or to other vesicles (Fig. 1b) such a compartmentalized system can additionally be spatially organized and its efficiency may be further enhanced (Fig. 1e). Crucial for us is that spatial structuring is beneficial to the first instance because the material exchange between reaction containers can be controlled and optimized. But additionally, we claim that spatial structuring enables to realize different reaction networks by one and the same set of chemical modules.