The 24 months deliverable of WP3 included the electrochemically induced ion-stimulated control of DNAzymes in gemlabs and lablets compartments. This deliverable has been addressed by the design of electrodes that include metal ion reservoirs as sponges. The potential-induced release of ions allowed the sequestered activation of DNAzymes. The following accomplishments were demonstrated:
(a) A method to electrically accumulate metal reservoirs on Au electrode surfaces was developed.
(b) The programmed potential release of one or two ions from the metal reservoirs associated with the electrodes was demonstrated by applying oxidative potential steps on the electrode. The potential range and the duration of the voltammetric pulse controlled the nature and content of the released ions.
(c) The released ions triggered-on catalytic transformations by the metal ion-induced activation of sequence-specific DNAzymes. The dose of the electrochemically released ions controlled the contents of the resulting DNAzymes.
(d) The electrochemical re-adsorption of the metal ions into the sponge electrode and the blockage of the DNAzyme activities were demonstrated. Also, the switchable and reversible release/re-adsorption of the metal ions was achieved.
Summary of experimental results:
1. The metal reservoirs on the Au surfaces were prepared by the electrochemical deposition of Pb2+ or Ag+ on Au surfaces.
2. Pulse voltammetric release of Pb2+ (potential step -0.6V to -0.2V vs. Ag wire quasi reversible electrode, QRE), or Ag+ (potential step -0.6V to 0.2V vs. Ag QRE), have activated the respective DNAzymes. Figure 1(A) depicts the dose-controlled activation of the Pb2+-DNAzyme. Similarly, the Ag+-cooperative stabilization of the hemin/G-quadruplex horseradish peroxidase-mimicking DNAzyme is depicted in Figure 1(B), where the electrochemical release of the Ag+ ions controls the activity of the hemin/G-quadruplex DNAzyme towards the oxidation of 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), ABTS2-, by H2O2 to the colored ABTS·– product.
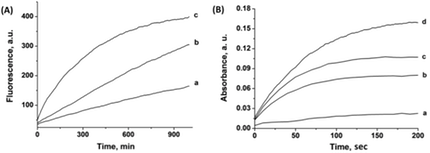
Figure 1. (A) Time-dependent fluorescence measurements, at λ=590 nm, corresponding to the electrochemical release of Pb2+ ions from a Pb-deposited Au surface. The solution contains a Pb2+-dependent DNAzyme functionalized with a fluorophore (ROX)/quencher (Black hole I) substrate. Pb2+ ions were released to the solution by the application of oxidative stripping pulses at -0.2 V vs. Ag QRE for: (a) 1 (b) 2 and (c) 3 seconds. (B) Time-dependent absorbance measurements, at λ=415 nm, corresponding to the electrochemical release of Ag+ ions from a Ag-deposited Au surface. The solution contains a catalytically inactive, Ag+-stabilized hemin/G-quadruplex DNAzyme. Ag+ ions were released to the solution by the application of oxidative stripping pulses at 0.2 V vs. Ag QRE for: (a) 0, (b) 0.1, (c) 0.2, and (d) 0.4 seconds.
3. The potential range of applied pulse controlled the nature of the released metal ion, resulting in the selective activation of the DNAzymes. Figure 2 depicts the “logic” activation of the respective DNAzyme. Upon applying a potential step -0.6V to -0.2V, only Pb2+ ions is released from the reservoir electrode, resulting in the activation of only the Pb2+-dependent DNAzyme, Figure 2(A). The application of a potential step -0.6V to 0.2V results in the concomitant release of Pb2+ ions and Ag+ ions. As a result, the Pb2+-dependent DNAzyme and the Ag+ ions-stabilized hemin/G-quadruplex are activated, Figure 2(B).
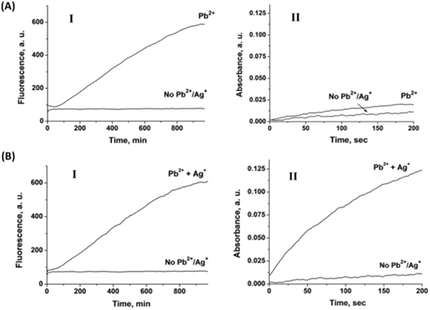
Figure 2. (A) Time-dependent fluorescence (panel I) and absorbance (panel II) measurements corresponding to the electrochemical release of Pb2+ ions from a Pb/Ag-deposited Au surface. (B) Time-dependent fluorescence measurements at λ=590 nm (panel I), and time-dependent absorbance measurements at λ=415 nm (panel II) corresponding to the electrochemical release of Pb2+ and Ag+ ions from a Pb/Ag-deposited Au surface. Fluorescence measurements were performed in the presence of Pb2+-dependent DNAzyme functionalized with a fluorophore (ROX)/quencher (Black hole I) substrate. Absorbance measurements were performed in the presence of a catalytically inactive, Ag+-stabilized hemin/G-quadruplex DNAzyme.
4. The cyclic electrochemical release/uptake of the metal ion sponges was demonstrated, Figure 3. By the cyclic potential-induced oxidative release of Pb2+ ions, the Pb2+-dependent DNAzyme was activated. Application of a negative potential reduced the Pb2+ ions and collected them on the electrode. By the cyclic application of oxidative/reductive potentials, the ON/OFF activation of the Pb2+-dependent DNAzyme was demonstrated.
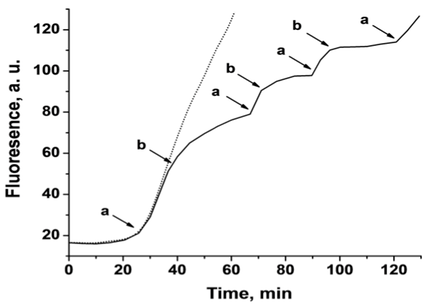
Figure 3. Time-dependent fluorescence measurements, at λ=590 nm, corresponding to the electrochemical release and uptake of Pb2+ ions from a Pb-deposited Au surface. The solution contains a Pb2+-dependent DNAzyme functionalized with a fluorophore (ROX)/quencher (Black hole I) substrate. Pb2+ ions were released to the solution by the application of oxidative stripping pulses at -0.2 V vs. Ag QRE at the times indicated by the (a) points. Pb2+ ions were reduced from the solution onto the electrode by the application of reductive pulses at –0.95 V vs. Ag QRE at the times indicated by the (b) points. The dotted line represents a single oxidation step, at the indicated point (a), of the Pb2+ ions from the surface and its subsequent fluorescence change.
Conclusions and perspectives:
The results demonstrate the first steps to construct artificial electronic cells mimicking complex cell functions. The electrical signals trigger on programmed catalytic processes, demonstrating branching of transformations and amplified chemical processes. The fact that one of the chemical transformations, the activity of the Pb2+-dependent DNAzyme, releases a strand, suggests that the appropriate design of the system may lead to DNAzyme catalytic cascades, ligation processes, fan-out reactions, and more. Such systems could provide very fundamental paradigms for future “living technologies”. Experiments toward these goals are in progress.